Motivation
First of all, it’s important to ask: why are we here? Why do we care about acids and bases? What’s the end goal? What would we like to be able to do once we’re done?
In short, the bonds between an electronegative atom and a hydrogen are very polarized, and thus are easy to break in polar solvents (such as water). As a result, such bonds break and form quite often and quite fast. So, if we’ll take a molecule such as aspartic acid, which is an aminoacid (a building block of proteins):
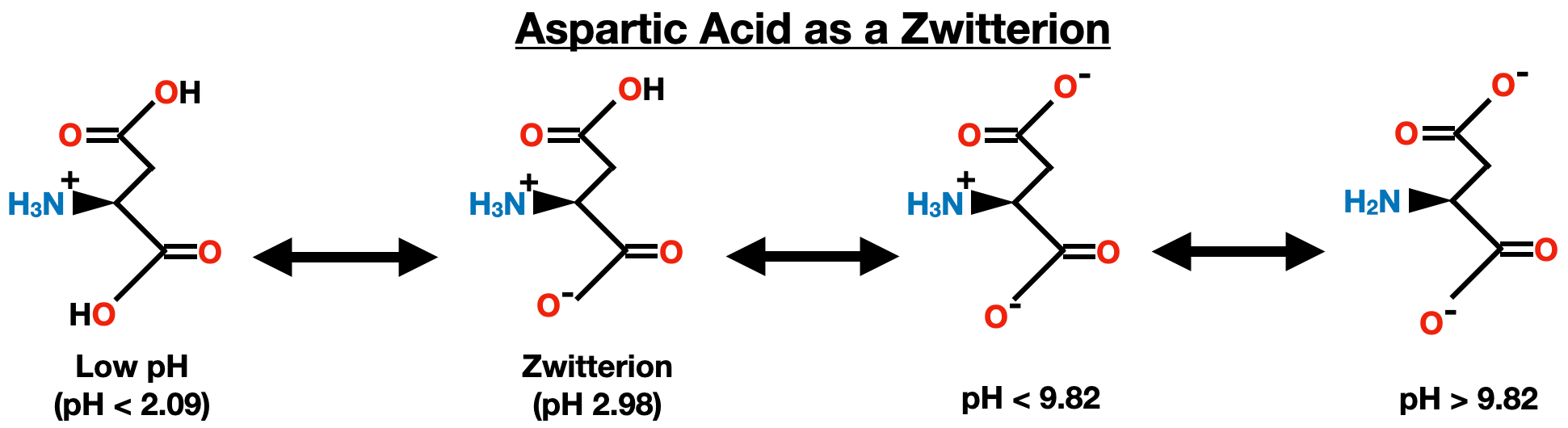
depending on some (unknown at the moment) factor the molecule may have hydrogens on both carboxylic groups (COOH) and on an amino group (NH3+), or only on an amino group and on one carboxylic acid, or only on an amino group, or nowhere else. Consider the carboxylic acid group which is on the top (farther from the amino group) — that’s the group which will not be bound to anything in a protein. If that group exists as COO- it can form very strong ionic bonds with other molecules. If that group exists as COOH, it can, at most, form a much weaker (than ionic bonds) hydrogen bonds. Those kind of interactions determine whether (or how strongly) a protein can bind a molecule. So, understanding in which form does COOH exist is essential for both fundamental study of proteins and for drug design. Of course there is a myriad other contexts in which we care whether a molecule is protonated (and if so, to what extent), but IIRC most of you have some interest in biology, so hopefully this example gives you a good enough reason to study acids/bases.
What do we need to know?
Historical Context
Historically, the chemistry as a science started with greater emphasis on inorganic compounds.
One might wonder: why? Why exactly did inorganic chemistry start to develop earlier than organic chemistry? Part of the reason why I enjoy writing such long notes even when I know that very few of them actually read them 🗿🗿🗿 is moments like this when I write something that I know and take to be true, but then I realize that I don’t know the why (I do have some guesses in this case), and so now I have a yet another fascinating thing to read about. Or, I could ask GPT4. Here’s the response: Why Inorganic Chemistry developed prior to Organic Chemistry?
One fun fact I do know (which is referenced by GPT4 albeit with a minor mistake) is that for a very long time, it was thought that organic molecules possess some spiritual element to it. The argument was: organic molecules come from living organisms, so they possess that which all living things have, and which differentiates them from non-living organisms. Some vital force. As a result, it was thought that organic molecules are fundamentally different from inorganic ones, and you cannot create the former from the latter. You can imagine the surprise then when in early 1800s Wohler reported a synthesis of urea (an organic molecule) from ammonium cyanate (an inorganic molecule). Although it’s common to refer to the Wohler synthesis as the downfall of vitalism, IIRC, it took a few more decades until Kolbe reported a synthesis of acetic acid (CH3COOH) from carbon disulfide (CS2) when the vitalism started to actually lose ground. I think it’s fascinating to realize that some of the things you subconsciously take for granted were subjects of hot debates a mere 200 years ago.
Subject Matter
Because inorganic chemistry was studied more extensively before organic chemistry, the first theory of acids and bases was, unsurprisingly, focused on inorganic compounds. That was the Arrhenius Theory of Acids and Bases.
Arrhenius theory was too rigid to be useful with organic molecules, so a different definition was due. That’s how Brønsted-Lowry Acid-Base Theory was developed. The sequence of acid-base theories was completed with Lewis Acid-Base Theory, which, quite interestingly, was published in the same year as the Bronsted-Lowry one, but conceptually may be considered as a generalization of the Bronsted-Lowry.
Now, to be honest, if you don’t know Arrhenius theory by now, I don’t think the marginal benefit of knowing it outweighs the time required to do so. And I don’t think Arrhenius theory is required to understand Bronsted-Lowry. As a result, I actually don’t see any reason to give you problems like Q3 from Section 5 Worksheet because I’d rely heavily on ideas from Arrhenius theory to solve it. So this time, I’m going to try something new and first show you how Question 3, Section 5 is solved and if you don’t understand why certain things are true, you can refer to the notes on Arrhenius theory.
With that in mind, here’s what I recommend you to do:
- Read solution to Question 3, Section 5
- Read notes on Arrhenius Theory of Acids and Bases. Treat it as a light-weight historical context for reference.
- Read notes on When Equilibrium meets Arrhenius. This should give you an introduction to pH, pOH, Ka/pKa, Kw.
- Carefully read notes on Brønsted-Lowry Acid-Base Theory. This is the most important topic both for this course and for your future. It also introduces pKb.
- Read a paragraph on Lewis Acid-Base Theory. This is mostly a light reading to have the full picture and have some anticipation for orgo.