If you read the notes on Arrhenius Theory of Acids and Bases, you might find that it provides a very narrow definition of the base: it must produce as an anion during dissociation. If you know anything about organic chemistry, you might realize that no organic molecule will ever satisfy that definition (although alcohols have an group, they cannot lose it through dissociation). This makes Arrhenius theory more or less useless for organic chemistry, which is not only of high interest to anyone who tries to study living organism, it constitutes the absolute majority of all possible molecules. So, we have to develop new theory.
A key observation and development upon Arrhenius theory, as hinted before, is that acids don’t actually dissociate. Instead of:
as proposed by Arrhenius, a proton transfer reaction happens between an acid and a water molecule:
Bronsted and Lowry proposed (quite amusingly - they did it independently of each other) to use the following definition of acids and bases:
- a Bronsted-Lowry acid is the molecule that donates a proton (an ion)
- a Bronsted-Lowry base is the molecule that accepts a proton (from an acid)
as a result, in reaction (2) the is considered an acid and is considered a base.
Where Things Get Ambiguous
There’s a very important aspect of the Bronsted-Lowry theory that you must not overlook. Let’s consider - the phosphoric acid. Within Arrhenius theory, we would write the dissociations as:
Within Bronsted-Lowry, these reactions become:
In the forward direction of (7) behaves as an acid that donates a proton to . However, we know that has a positive pKa even for the first stage (to be precise, pKa), so not all of will be converted into . As a result, there’ll be a dynamic equilibrium, and we can have a reverse reaction to reaction (6):
In this reaction, the proton is transferred from to . So, by definition, the is an acid, and is a base.
But hold on, we said was an acid just a couple of sentences ago. Is it an acid or is it a base? It’s both. The classification depends on the reaction in which participates. If it participates in a reaction in which it donates a proton, as in (7), then it acts as an acid; if it participates in a reaction in which it receives a proton, as in (6r), then it acts as a base.
In Arrhenius theory, the classification was rigid and permanent: something is either an acid, or a base. If something is an acid, it will always be an acid, day or night. Simply because that definition is tied to the structure of the molecule: does it have that can be easily lost as ? The definition of Bronsted-Lowry is inherently attached to the process: we can only assign labels acid or base when two molecules excange a proton. In absence of such proton transfer, we could only say whether a molecule is capable of being an acid or a base. If it has a proton that can be easily lost, then it can behave as an acid if a sufficiently strong base will be present. If a molecule has a lone pair that can attach , then it can behave as a base if a sufficiently strong acid will be present.
How do I know if a molecule can behave as an acid or as a base?
One of the most important ideas in Chemistry (and Biology) is that function is determined by structure. So if we want to know if something can behave as an acid or as a base, we must know the structure of a molecule. And the structure is not just the molecular formula. Consider these two molecules:
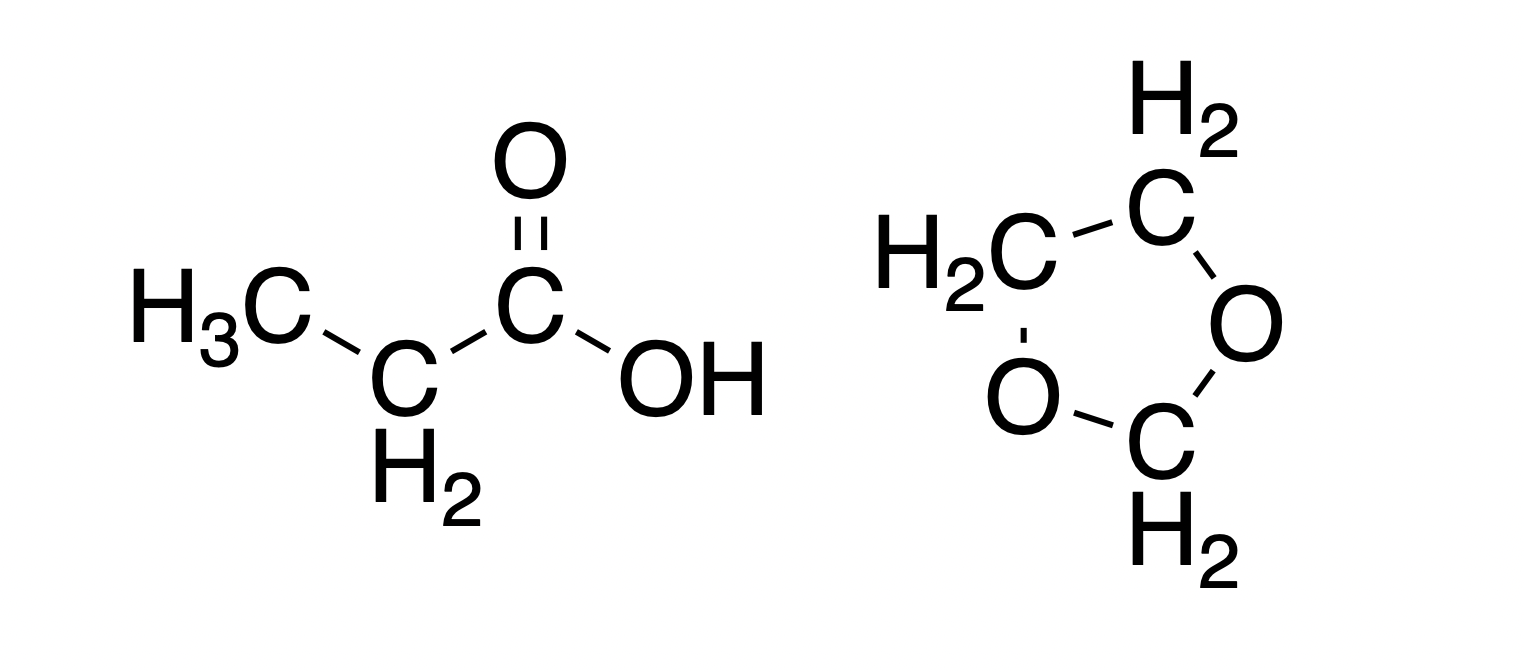 Both of them have a molecular formula of . However, only the molecule on the left is capable of being an acid (in fact, the molecule on the right is capable of being a relatively weak base). Such importance of knowing the structure of a molecule is part of the reason why Bronsted-Lowry theory is often taught within the Organic Chemistry course/textbook
Both of them have a molecular formula of . However, only the molecule on the left is capable of being an acid (in fact, the molecule on the right is capable of being a relatively weak base). Such importance of knowing the structure of a molecule is part of the reason why Bronsted-Lowry theory is often taught within the Organic Chemistry course/textbook
Yes, I also struggle to see a reason why you’re being taught Bronsted-Lowry within GenChem. I guess the main desired outcome is to give you proficiency with pH, pOH, pKa, and pKb, but one could easily argue you could easily achieve it even within Arrhenius theory.
Also, this means that with a very high probability, you’re will not be expected to be able to determine whether something can behave as an acid or as a base. What’s more likely to be tested is whether you can see whether something behaves as an acid or as a base in any given reaction, which is much simpler and the only thing you need to do is to determine who donates and who accepts a proton.
But, if you’re curious, I can tell you what to do if you have a structure of a molecule. How do I know, for example, that the can behave as an acid? I look for a hydrogen that has a significant partial positive charge. In other words, I look for a hydrogen that is attached to some atom that is more electronegative than hydrogen. In such a bond, the more electronegative atom will attract electron density closer to itself, leaving with less electron density. This is what gives the atom a partial positive charge, and it also makes it easy to abstract the proton away. The strength of a chemical bond comes from electrons being shared in a common space around two atoms, effectively gluing them together. If most of the glue is attached to just one atom, there’s not a lot of force keeping another close to it.
So, in practice, you could abstract a hydrogen if it’s attached to N, O, S, Cl. Sometimes even you could abstract it from carbon, if that carbon is electron-deficient itself. But that’s an orgo territory. In this course, I imagine the organic acids will be limited to carboxylic acids .
How do I detect a base? I look for lone pairs. What is a lone pair? Two electrons that are located on an atom and are not shared in some bond. How do I know if there’s a lone pair? Here’s a primer:
- C (carbon) has 4 valence (outer-shell) electrons. It’s common for a carbon to form 4 bonds by giving out one electron into each bond. As a result, carbon with 4 bonds (e.g. ) doesn’t have a lone pair. Carbons with three bonds may exist in two flavors (without lone pair) and (with a lone pair), but they’re definitely orgo territory.
- N (nitrogen) has 5 valence (outer-shell) electrons. It’s common for N to form 3 bonds by giving out one electron into each bond. The 2 remaining electrons reside as a lone pair on nitrogen. e.g. (the colon shows the lone pair). Sometimes nitrogen can donate this lone pair to form a molecule with 4-bonds and a positive charge: (there’s no lone pair). Sometimes a strong base can take away hydrogen from nitrogen, leaving only two bonds and two lone pairs: .
- O (oxygen) has 6 valence (outer-shell) electrons. It’s common for O to form 2 bonds by giving out one electron into each bond. The 4 remaining electrons reside as two lone pairs on oxygen. E.g. oxygen in water has 2 lone pairs . Sometimes oxygen can donate thi slone pair to form a molecule with 3 bonds and a positive charge (one lone pair on oxygen left). Sometimes a strong base can abstract one hydrogen, leaving oxygen with one bond and three lone pairs: .
In sum, you’ll most likely only see carbon with 4 bonds, which doesn’t have lone pairs, so it cannot behave as a base. The nitrogen with three bonds has a lone pair and so can act as a base or (R is an arbitrary group). If one of the three bonds to nitrogen is with hydrogen, it can also act as an acid. The following reaction is entirely possible:
albeit rare (you need a really strong base). The oxygen with two bonds has a lone pair and thus can act as a base acts as a base to form . Notably, if one of the bonds is to hydrogen, it can also act as an acid: can act as an acid to become .
How do I know if a molecule will behave as an acid or as a base?
Let’s consider and . Let’s first consider the case when an ammonia behaves as a base and water behaves as an acid. We might have the following equilibrium:
We might wonder in which direction is the equilibrium shifted: does ammonia in aqueous solution exist as an or as a ? This question can be reduced to the question of which proton transfer reaction dominates: (9f) or (9r)?
If you think about it, the question is equivalent to which molecule is a stronger acid or ? With this formulation, you might see the path to the answer: we just need to compare pKa of to the pKa of . The pKa of water is 15.7 (will you be amused if I tell you that you can get this value if you divide by 55.55 and then take the negative log of the result?) while the pKa of is 9.25. Because the pKa of ammonium ion is lower than the pKa of water, ammonium ion is a stronger acid, so 9r dominates and so most of the ammonia is present as (this is precisely the reason why is a weak base within Arrhenisu theory).
We can get to the same conclusion in a more mathematic way. Let’s write the equilibrium constant for reaction (9):
Let’s write the Ka’s for water and ammonium ion:
Let’s see what happens if we divide equation (11) by (12):
you might notice that we get exactly the expression (10), i.e. the equilibrium constant!
You know that if for reaction (9) then the equilibrium will be shifted towards products. If , then the equilibrium will be shifted towards reactants. From (13) we see that the value of is determined by the relative values of of an acid in the forward reaction and an acid in the reverse reaction. Because water has greater pKa, it has lower Ka, so K<1, and the equilibrium is shifted towards reactants. Just as we predicted above.
A few notes on conjugate terminology
Let’s go back to equation (9):
Quite often, you’ll see the following terminology: is a base, is a conjugate acid, is an acid, is a conjugate base.
If people were precise, they would have said: in the forward reaction acts as a base and acts as an acid, forming and . This reaction is reversible, so the proton transfer may occur in the reverse direction, i.e. may act as an acid, but to avoid confusion with , we will call a conjugate acid and will be called a conjugate base.
Please note that if we were to write equation (9) as:
(which we’re perfectly allowed to do, there’s nothing which mandates writing it one way or another), the whole terminology would be swapped: now acts as an acid, acts as a base, acts as a conjugate base, and acts as a conjugate acid.
So what’s the point you may ask? The point is that with this terminology (let’s stick to equation 9), the question of whether the forward or reverse reaction dominates can be formulated as which is stronger: the acid (i.e. ) or the conjugate acid (i.e. )? If the acid is stronger, the forward direction dominates. If the conjugate acid is stronger, the reverse direction dominates.
To sum up, you could either look at the equilibrium (9), write out both directions explicitly as we did above (9f and 9r) and consider them as separate reactions, identify acid in both, compare the strengths of each acid, and then make conclusions (without ever using the conjugate terminology) OR you could look at (9), identify acid and the conjugate acid, and compare those. Whatever makes you feel comfortable.
Introducing Kb and pKb
A Bronsted-Lowry acid is a molecule that donates a proton. If we write the reaction of an acid with the solvent:
We can write an expression for the equilibrium constant:
and noting that the concentration of the solvent is approximately constant, we can define:
The definition of may be, as I suspected in When Equilibrium meets Arrhenius, a purely historical artifact. Regardless, we use as a measure of the strength of an acid.
In the Bronsted-Lowry theory, we have a new definition of a base: a molecule that accepts a proton. Let’s write a general equation for the reaction of a base with a solvent:
Similarly, we can write an expression for the equilibrium constant:
and again, in a similar manner, we can note that the concentration of the solvent is approximately unchanged, and so we can define:
and so we can use this (or alternatively the negative log of it, i.e. pKb) as a measure of the strength of a base.
Do we get new information from Kb?
Let’s consider , i.e. ammonia as our base. Let’s plug this into equation 18:
Oh, hello equation (9). This means that if we wanted to know whether this equilibrium is shifted towards reactants or products, we could have just looked at the pKb of ammonia. It’s equal to 4.75, meaning it’s a fairly weak base, so only a fraction of ammonia will be protonated, and we’re done. Cool.
We can actually show that Kb of ammonia is just the Ka of NH4+ in disguise. Let’s write the Kb for ammonia:
Let’s now divide both sides by :
Let’s recall that and make this substitution in the right hand side only:
Take a look at equation (12). Do you notice that the right hand side is just ?
I guess this is another place in which the conjugate terminology gets useful. Eq (24) shows that the basicity of a base is directly related to the acidity of the conjugate acid of that base. Ugh, the terminology may not be that useful after all. Let’s do some manipulations with eq. (24): particularly, take the negative log of both sides:
Eq 25 shows that the lower the pKa of the , the greater the pKb of and vice versa. In other words, if is a stronger acid, is a weaker base. This should make sense to you: a strong acid loses a proton easily. For that to be true, the molecule which forms when the acid loses a proton should not be able to easily abstract a proton back. In other words, it should be a weak base.