Q: What happens to the color of alizarin between 7 and 11?
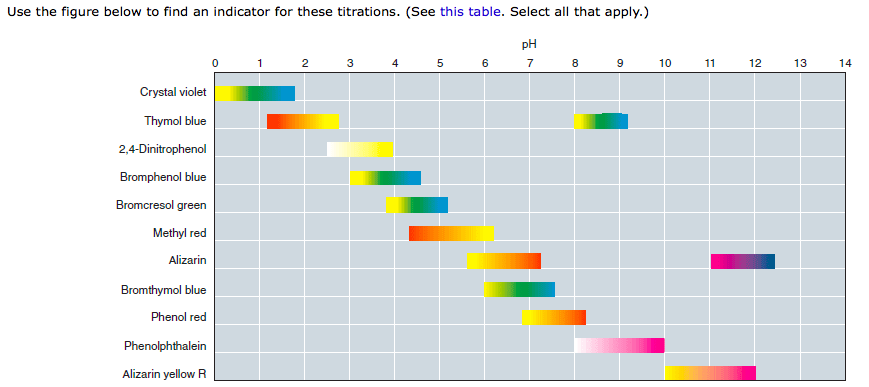
It was quite a lmao moment for me in class because … this must be an erroneous figure. Thinking from first principles, if you claim that a molecule has red color at pH7 and purple at pH 11, you must have some explicit color change; in other words, there should be another gradient bar between pH 7 and pH 11. Or, maybe, simply, the described behavior for alizarin is wrong.
If you try to do some quick google search, you might find another version of the table
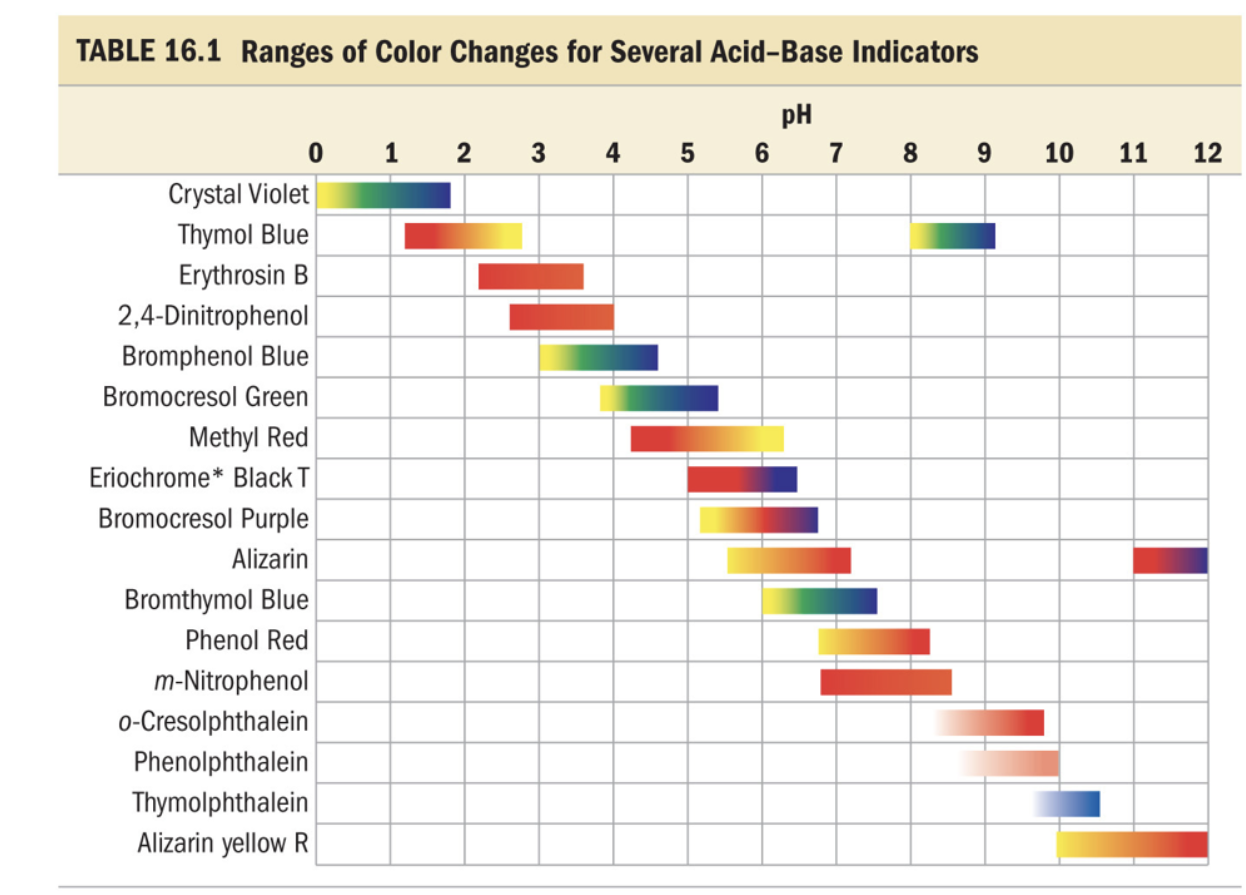
Which claims that the color at pH 11 is not pink but red. Which would make sense.
You can also find a different figure, though:
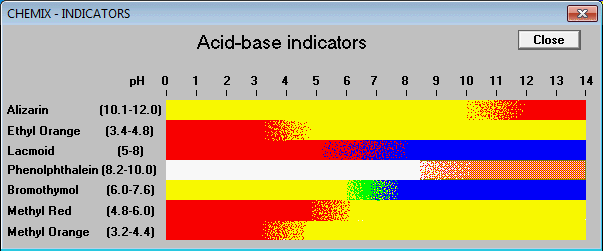
and yet another one
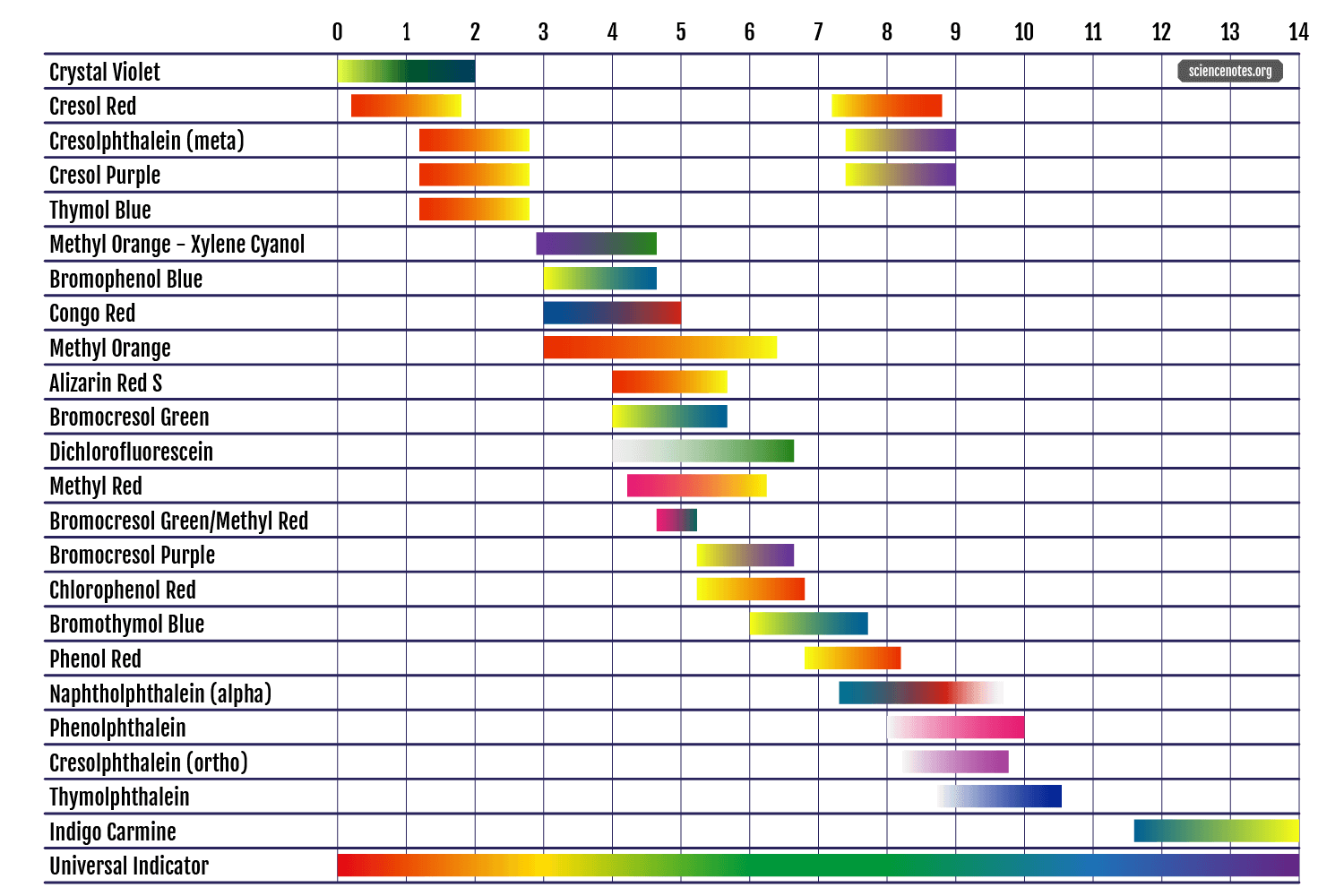
notice how the color change at pH 5 is from red to yellow, not from yellow to red as before.
So, which is one correct? I don’t know, but I also don’t really care enough to spend my time trying to figure it out. I’ll leave it to whoever wants to defend the accuracy of one of the figures above.
There are 2 takeaways:
- color never jumps. If alizarin does indeed undergo 2 transitions, the final color of the first transition must be the same as the starting color of the second transition.
- a lot of scientific content online is erroneous. never take anything for granted.
What do LLMs say
Let’s ask three different LLMs what do they know about color transitions in alizarin.
The prompt is the same for all: What color changes does alizarin undergo upon varying pH from 0 to 14? Enumerate all color transitions present
GPT4 (chat.openai.com)
Alizarin, a historically significant dye and a prototype for phthalein dyes, undergoes color changes across the pH spectrum due to the ionization of hydroxyl groups and the formation of different ionic species. Its structure contains hydroxyl groups that can donate or accept protons (H⁺ ions) depending on the pH of the environment, leading to structural changes that affect its light absorption properties and, consequently, its color.
-
pH 0 to ~4 (Acidic Range):
- At very low pH (close to 0), alizarin exists primarily in its neutral form, which is not ionized. The color in this range is typically yellow.
- As the pH increases but remains in the acidic range, alizarin starts to lose protons from its hydroxyl groups, leading to the formation of a monoanionic species. This transition begins to change the color towards red.
-
pH ~4 to ~6 (Transition Range):
- In this pH range, alizarin further ionizes to form a monoanionic species more prevalently. This transition is associated with a deeper red color.
- The exact pH value where this transition occurs can vary slightly depending on the specific conditions (e.g., solvent, temperature).
-
pH ~6 to ~10 (Neutral to Basic Range):
- As the pH moves into the basic range, alizarin loses another proton to form a dianionic species. This species absorbs light differently, leading to a shift in color towards purple or violet.
- This shift indicates the presence of a resonance-stabilized structure that can absorb and reflect different wavelengths of light.
-
pH >10 (Highly Basic Range):
- In highly basic conditions, alizarin can form more complex ionic species, potentially leading to further color changes. However, the most notable transition is towards the purple or violet range as the dianionic form prevails.
- The specific changes in color at very high pH values might be less pronounced than those observed in the acidic to neutral range.
Structural Changes Responsible for Color Transitions:
- Protonation and Deprotonation: The primary structural changes involve the gain or loss of protons (H⁺ ions) by the hydroxyl (–OH) groups in alizarin. This process alters the electronic structure of the molecule, affecting its conjugation and the distribution of electrons across the molecule.
- Conjugation: Changes in the degree of conjugation affect the energy levels of the electronic states of the molecule. Increased conjugation lowers the energy gap between the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO), shifting the absorption of light to longer wavelengths (bathochromic shift), which changes the color observed.
- Ionic Species Formation: The formation of monoanionic and dianionic species under different pH conditions changes the resonance structures and electronic delocalization within the molecule, directly influencing its optical properties and the colors it exhibits.
These color transitions and structural changes are fundamental principles in the study of dyes and pigments, illustrating how chemical properties can influence the visual characteristics of compounds.
Gemini (gemini.google.com)
Absolutely! Alizarin is a fascinating compound because its color transformations directly reflect structural changes due to pH. Here’s the breakdown of its color changes and the structural reasons behind them:
pH Ranges and Color Changes:
- pH 0-5.2: Alizarin remains in its neutral molecular form and appears yellow.
- pH 5.2-10: The first hydroxyl group of alizarin deprotonates (loses a hydrogen ion). This changes the molecule’s electronic structure, causing it to absorb different wavelengths of light and appear orange-red.
- pH 10-12: The second hydroxyl group also deprotonates. This further modifies the molecule’s ability to absorb light, resulting in a shift to a striking violet color.
- pH >12: At very high alkalinity, alizarin begins to degrade and lose its color intensity.
Structural Changes and Color
The key to alizarin’s color transformation lies in its two hydroxyl groups (-OH) attached to its anthraquinone core. Let’s break down the changes:
-
Neutral Form (Yellow): In its neutral form at low pH, the hydroxyl groups form hydrogen bonds. This keeps the electrons in the molecule somewhat localized, leading to absorption of light primarily in the blue region of the spectrum, hence the yellow appearance.
-
Deprotonation (Orange-Red and Violet): As pH increases, the hydroxyl groups become increasingly deprotonated (lose their H+). This creates negatively charged oxygen atoms (alkoxides) that are far more able to interact with the rest of the molecule’s electron system. This alters the distribution of electrons within the molecule, leading to a shift in the wavelengths of light absorbed.
Why the Different Colors?
The extent of deprotonation determines the exact color:
- One Deprotonation: Orange-red color is due to the altered electron distribution and a greater ability to absorb green light.
- Two Deprotonations: The violet color arises from even greater modification of the electron system, where alizarin now absorbs both yellow and green light.
Important Note: Alizarin is an example of a pH indicator. These substances change color in response to distinct changes in acidity or alkalinity, making them useful tools in chemistry.
Let me know if you want to dive deeper into the electronic transitions or other aspects of pH indicators!